Pdi Prevanticsö (10137_S40750)
Important Purchase Information: Please ensure you select the correct Choose Product Details and Unit of Measure (UoM) before adding this item to your cart.
Note: Product images may vary slightly by variant.
Pdi Prevanticsö (10137_S40750)
Important Purchase Information: Please ensure you select the correct Product Option and Unit of Measure (UoM) before adding this item to your cart.
Note: Product images may vary slightly by variant.
Description
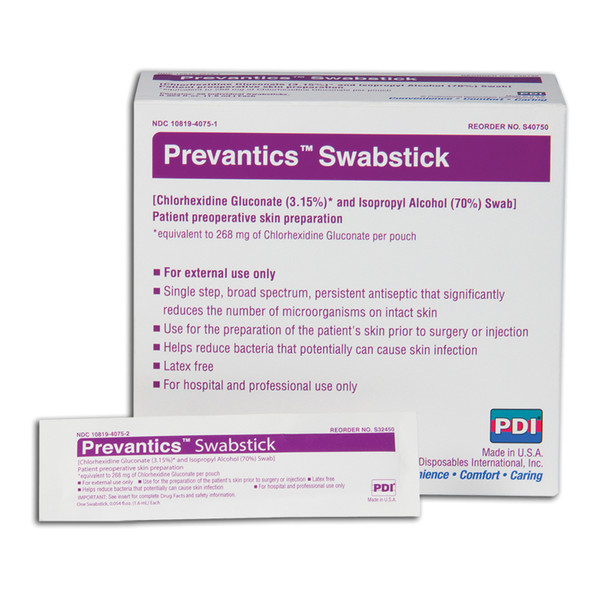
The first FDA approved 3.15% Chlorhexidine Gluconate and 70% Isopropyl Alcohol skin antiseptic. Approved or pre-operative* or pre-injection applications. Meets skin antisepsis guidelines from organizations such as CDC and APIC . All Swabsticks are clinically proven to have persistent antimicrobial activity up for up to 7 days.* Premoistened, ready to use applicators contain no glass and do not require activation before use. Swabsticks feature unique two sided applicators for easy prepping around joints, interdigital areas and skin folds. *Swabstick and Maxi Swabstick only.
Specifications
Accepted Licenses
For Business Accounts:
- Pharmacy or wholesale distributor license issued by the state board
- License address must exactly match the shipping address
- License must be from the same state as the shipping address
For Individual Practitioners:
- Doctor, dentist, or nurse practitioner license issued by the state board
- Address does not need to match, but license must still be from the same state as the shipping destination
Note: A DEA license will not meet the requirement.
Please email the appropriate license to support@bttnusa.com so that we can confirm your eligibility to purchase pharmaceutical products.